STOP CASSINI
A logical comparison of anti-personnel land-mines and plutonium particles
By Dr. Ross Wilcock
"Landmines for Cells"
A particle of plutonium 239 revealed by autoradiography. The black star in
the middle of the picture shows tracks made by alpha rays emitted from a
particle of plutonium 239 in the lung tissue of an ape. The alpha rays do
not travel very far but once inside the body they can penetrate more than
10,000 cells within their range. This set of alpha tracks (magnified 500
times) occured over a 48 hr period. The plutonium 239 particle that emitted
them has a half life of 24,400 years. [Lawrence Radiation Laboratory,
Berkeley California, September 20, 1982.]
Note that Pu238 (half life of 89.7 years) is 272 times hotter (more active)
than Pu239. It decays much faster so it's biological effects are stronger
in proportion. Pu decay pathways are all radioactive.
1 kg. Pu238 was released from a navigational satellite launch accident over
the Indian Ocean in 1964. The plutonium dispersed remains detectable in the
southern hemisphere.
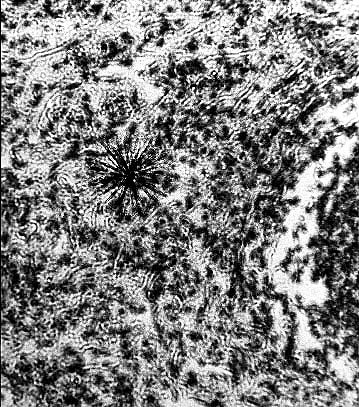 This illustration is taken from Robert Del Tredici's book Working in the
Fields of the Bomb - published in 1987. It shows a
plutonium particle emitting ionising radiation. The tracks are about 35
microns (5 cell diameters) but this is a two dimensional view of
a 3D tissue event, which in fact occurs continuously in biological space.
Like a land mine that never stops exploding, it is perpetually
damaging and destroying cells.
This illustration is taken from Robert Del Tredici's book Working in the
Fields of the Bomb - published in 1987. It shows a
plutonium particle emitting ionising radiation. The tracks are about 35
microns (5 cell diameters) but this is a two dimensional view of
a 3D tissue event, which in fact occurs continuously in biological space.
Like a land mine that never stops exploding, it is perpetually
damaging and destroying cells.
Since it has a short emission path - it is difficult to detect outside the
body. Customary histology and autopsy studies do not detect it
so plutonium in the living body is likely to be an occult, undetected and
unreported factor. In man, it is particularly well situated to
cause harm since it lodges especially in the body-wide reticulo-endothelial
system. Here it can impair and kill cells of the immune
system leading to immune deficiency and eventually leukemia. Leukemia is
found down wind from Winscale which has been
processing nuclear waste and extracting plutonium since 1952.
Winscale/Sellafield is still pumping "nuclear effluent" into the Irish
Sea every night. Much of this is carried by Northeast Atlantic currents up
the coast of Norway to the Arctic Ocean where it accounts
for 20% of measured current Arctic plutonium pollution. There was an
incident at Sellafield when the cooling system failed and divers
found the water intake plugged with monster jelly fish. Fishing is
forbidden close to Sellafield where there are high environmental
plutonium levels in Irish Sea sediment.
The first plutonium bomb (Fat Man, Nagasaki, August 1945) comprised 15 kg
of Pu239. Of this only 1.5 kg fissioned, and the
remainder (13.5 kg) was vaporized. It has been shown that within two years
the Nagasaki plutonium became evenly distributed
around the northern hemisphere and random sampling in stable ice layers in
the Canadian Arctic for instance showed traces. It is
inferred that from this time, Pu239 could gain access to biological systems
and to the food chain. Some plutonium would be found in
sediments, but it forms water soluble hydroxyls, and hydroxy halides which
can be absorbed into living systems.
Plutonium has been shown to gain access to marine life and food chain in
varying proportions. It is taken up most actively by
plankton, and is also rapidly excreted by them, but plankton feeders such
as fish larva, herring, pilchard, anchovy and whales are
susceptible to ingesting it. It is also taken up significantly by bottom
feeding marine creatures that live on or disturb sediment. These
include starfish, octopus, flatfish and cod. Effects on fish might include
reduced fecundity, deformities, increased disease
susceptibility and reduced longevity. This would be seen as falling size
and numbers of fish that is - loss of ocean bounty. World
fishing accounts for double the food mass of land husbandry.
It hs also been shown to become incorporated into tree rings. Plutonium
entry into edible plants appears possible but evidence is hard
to find. Plutonium is so biotoxic that its chemical toxicology - which
would probably resemble lead - is overshadowed by its
radiotoxicity.
There are of course other radioactive products produced by nuclear
technology. The most studied is radioactive iodine with a relatively
short half-life. Strontium and cesium have been studied. Others may be
equally significant but hard to study or pin down. Strontium
was incorporated into the dental enamel of childrens' teeth during the time
of atmospheric testing. Each test produced a detectable
peak.
It is customary for nuclear physicists to speak in terms of half-life.
However, when the whole decay pathway for say Plutonium 238
is examined - around 12 radioactive daughter products are produced
including Radium and Radon. There is extensive documentation
of biological effects of these. Biological effects can be expected from the
whole decay pathway. [See
Decay Simulation ]
"The unleashed power of the atom has changed everything save our modes of
thinking, and thus we drift towards unparalleled catastrophe. Concern for
man himself and his fate must always form the chief interest of all
technical endeavours... Never forget this in the midst of your diagrams and
equations." Albert Einstein, physicist.
Some Serious Questions:
When it comes to "nuclear demining" - such a task would seem to be
impossible. A landmine demining program is daunting enough. What can be
done to recover such biologically harmful material from the environment?
How can these materials be detected in living tissues?
How can they be extracted from the living body? - from yourself?
Is there any possibility of remediation for anticipated harmful effects
once they are recognized?
Where are the "at risk areas"? - Places where nuclear materials are mined
and processed; military sites; storage sites; testing sites; nuclear
reactors (80% of Soviet nuclear reactors were built close to geological
fault lines); nuclear processing plants.
Should the living oceans of the world be treated like a sewer? Until Man
began to interfere - they were teeming with life. Many nations are dumping
unwanted hazardous materials in the seas. Is this wise? Nature is highly
adept at recycling - what goes down is likely to come up again in the water
cycle, or the food cycle though it may take a thousand years. Is it wise to
lay up trouble for the future - until a storm stirs up the toxic sediments?
Why is there so little information and understanding about occult elements
in foodstuffs?
Why are human remains and other species remains not routinely checked or
sampled for lifetime total heavy metal burden?
Might there be a quantitative threshold for individuals and for living
populations in relation to these matters? - What happens when a threshold
is exceeded?
How is it possible to justify the NASA Cassini project to Saturn planned
for early October 1997 which will be carrying 33 kg of Pu238 intended for
electricity generation but placing at unprecedented risk the integrity of
many lives on earth - including inumerable unborn - in the event of an
unplanned misadventure.
Does anyone have a clear understanding of the global ramifications of these
many difficult problems potentially affecting all species, and all progeny?
Will people of future generations look back on these times with horror for
what we did to the bountiful inheritance of Nature? Will the verdict of
history be ignorance, willful folly or crime? As these kinds of problems
accumulate, how long can we hope that Life on Earth will continue?
by Dr. Ross Wilcock
References:
1. From At Work in the Fields of the Bomb, Robert del Tredici, Harper and
Row, 1987, page 39.
2. Plutonium in the Environment Symposium Report,
Ottawa, July 1994:
http://www.elsevier.nl/estoc/publications/store/3/09698043/SZ955324.shtml
3. Environmental Plutonium in Humans by David M. Taylor (see Plutonium in
the Environment)
4. Complimentary material is available at:
http://www.pgs.ca/pages/nlnucleg.htm
Dr. Ross Wilcock
rwilcock@web.net
http://www.pgs.ca/
Home page of this article...
http://www.pgs.ca/pages/nl/lmcells.htm
Reprinted online by permission of the author.
This article has been presented on the World Wide Web by:

The Animated Software Company
http://www.animatedsoftware.com
 rhoffman@animatedsoftware.com
rhoffman@animatedsoftware.com
Last modified February 27th, 1999.
Webwiz: Russell D. Hoffman
 This illustration is taken from Robert Del Tredici's book Working in the
Fields of the Bomb - published in 1987. It shows a
plutonium particle emitting ionising radiation. The tracks are about 35
microns (5 cell diameters) but this is a two dimensional view of
a 3D tissue event, which in fact occurs continuously in biological space.
Like a land mine that never stops exploding, it is perpetually
damaging and destroying cells.
This illustration is taken from Robert Del Tredici's book Working in the
Fields of the Bomb - published in 1987. It shows a
plutonium particle emitting ionising radiation. The tracks are about 35
microns (5 cell diameters) but this is a two dimensional view of
a 3D tissue event, which in fact occurs continuously in biological space.
Like a land mine that never stops exploding, it is perpetually
damaging and destroying cells.